Clinical Trials Services
At Adhrysa Lifescience, we specialize in providing comprehensive clinical trials services designed to support pharmaceutical companies, biotechnology firms, and healthcare organizations in advancing medical research. Our clinical trials are conducted with the highest standards of accuracy, patient safety, and regulatory compliance, ensuring reliable results that contribute to the development of innovative therapies and treatments.
- Preclinical Study: is one of the most important stages in the development of new drugs or treatments, conducted before clinical trials in humans. It involves laboratory, invitro and in-vivo testing to evaluate the safety, efficacy, and potential toxicity of a drug or medical device. The key aspects of this study is Safety Assessment, Efficacy Evaluation, Pharmacokinetics, Pharmacodynamics, Regulatory Compliance
- Phase I-IV Clinical Trials: We offer end-to-end management for all phases of clinical trials, from early-stage human testing (Phase I) to large-scale studies (Phase III) and post-marketing surveillance (Phase IV).
- Regulatory Compliance: Our team ensures that all clinical trials adhere to national and international regulations, including FDA, EMA, and ICH-GCP guidelines, to guarantee ethical practices and data integrity.
- Patient Recruitment and Retention: We leverage advanced patient recruitment strategies and retention programs to ensure timely and successful trial completion.
- Data Management and Biostatistics: Our state-of-the-art data management systems and biostatistical analysis ensure accurate, insightful data that drives clinical trial success.
- Site Selection and Monitoring: We assist with selecting the best clinical trial sites and provide ongoing monitoring to ensure protocol adherence and trial integrity.
- Quality Assurance: Our clinical trials undergo rigorous quality control procedures to ensure the reliability and safety of our findings.
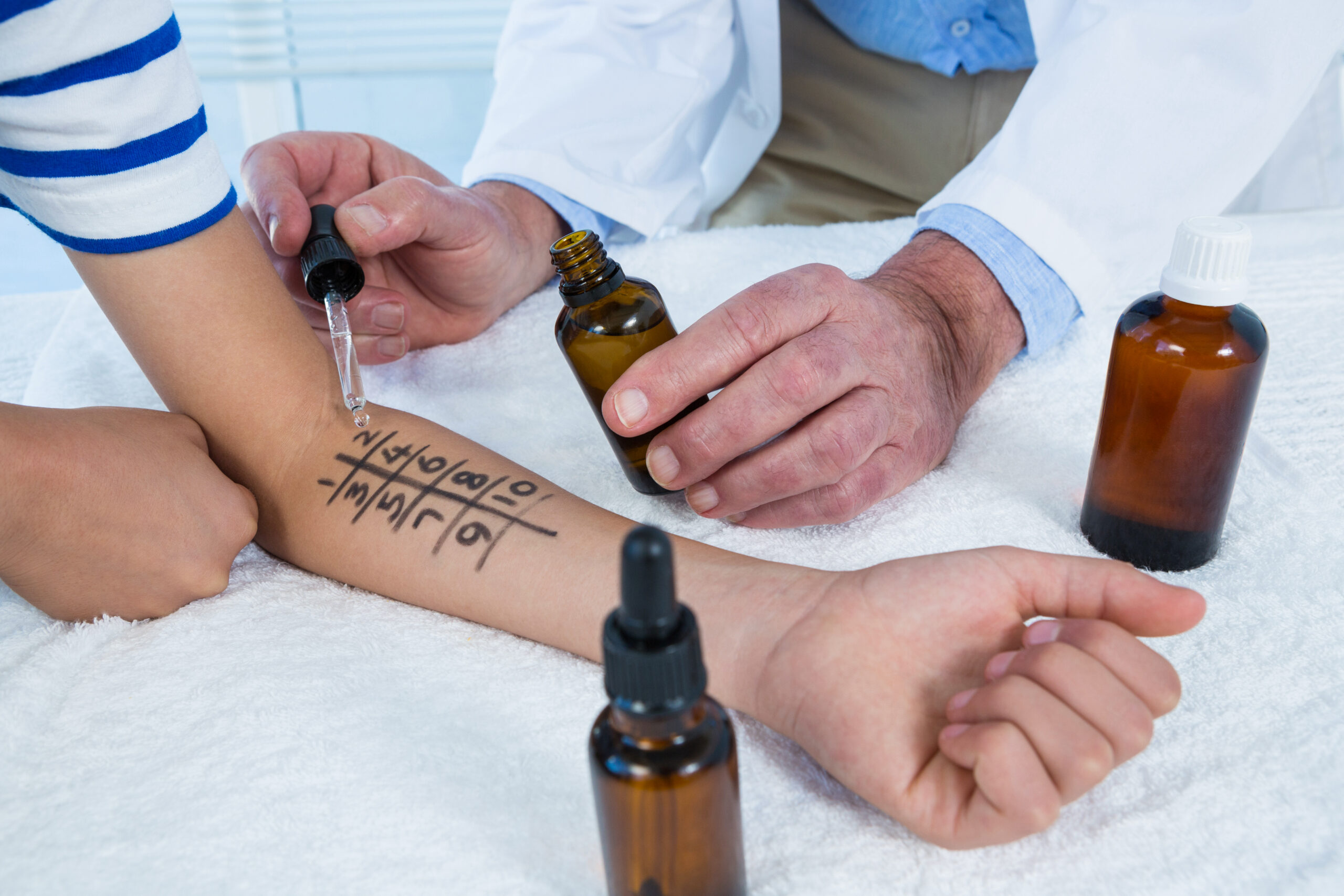

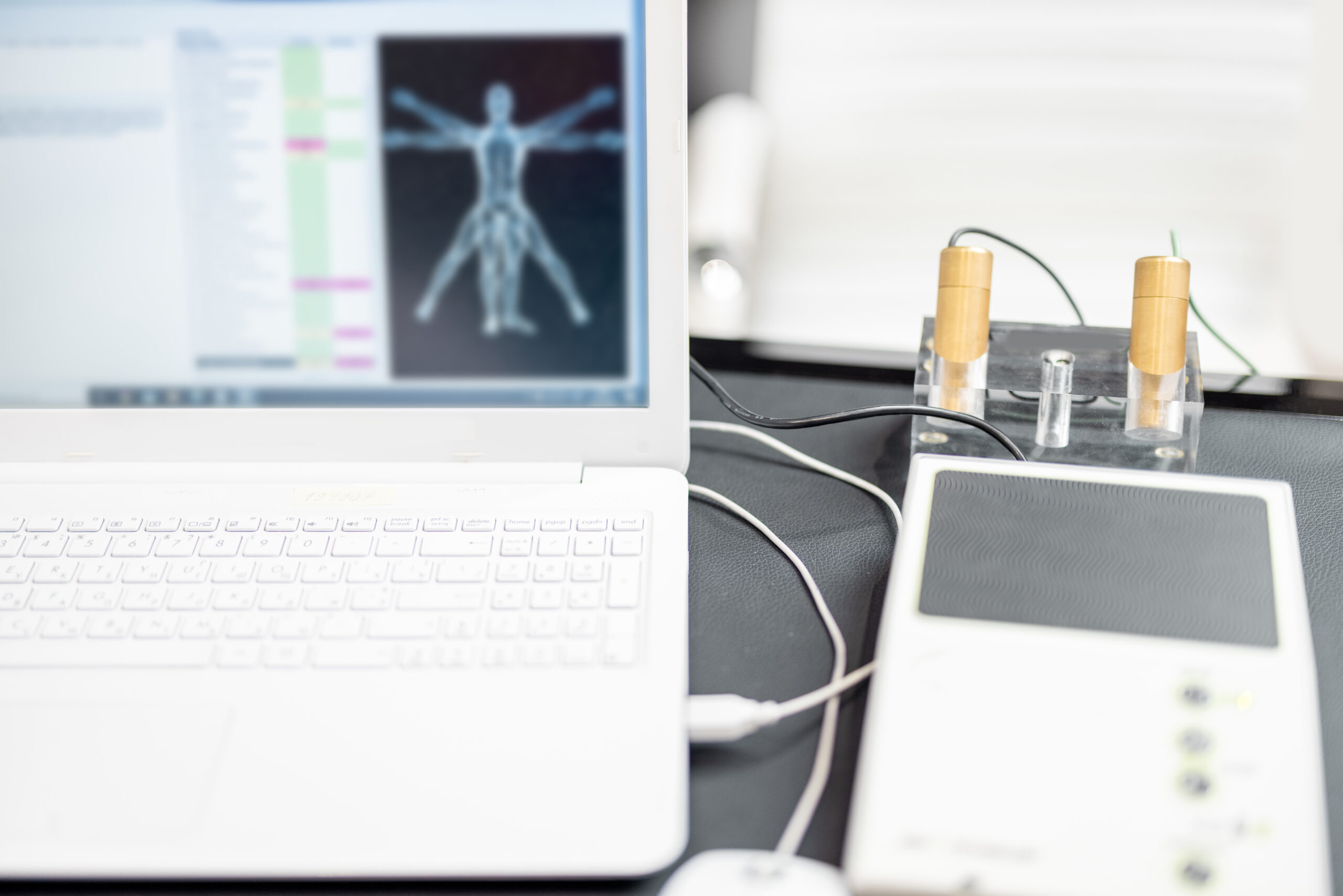
Pre-Clinical Study
Our preclinical studies provide a crucial foundation for advancing your products through the research pipeline. We conduct thorough preclinical investigations, including safety assessments and efficacy evaluations, to support the development of promising candidates and ensure their potential for success.
Read More
Herbal Clinical Trials
At Adhrysa we focus on evaluating the safety and efficacy of herbal remedies and formulations.Our team works closely with you to design and conduct rigorous studies, ensuring that your herbal products meet regulatory standards and satisfy consumer expectations.
Read More
Nutraceutical Clinical Trials
We conduct extensive clinical trials for nutraceuticals to verify their efficacy and safety. Our research expertise supports you in substantiating health claims and launching innovative nutraceutical products in the market.
Read More
Cosmeceutical Clinical Trials
We specialize in cosmeceutical clinical trials, supporting the development of skincare and cosmetic products. Our research validates product claims, enhancing consumer trust and boosting competitiveness in the beauty and skincare industry.
Read More
Project Management
We excel in meticulous clinical trial management, ensuring smooth execution through every phase. Our dedicated team oversees planning, implementation, monitoring, and reporting, adhering to the highest industry standards.
Read More
Medical Writing & Review
Our specialized services cater to various medical writing needs of the pharmaceutical, biopharmaceutical, and biotechnology industries throughout the product lifecycle, ensuring precision and adherence to industry standards, whether it is an official document or a scientific paper.
Read More